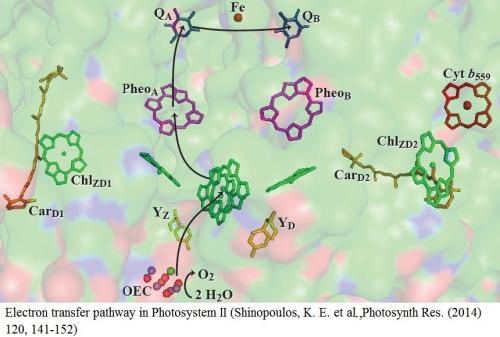
 The enzyme photosystem II (PSII) catalyzes the light-driven oxidation of water in all of oxygenic photosynthesis. The active site of PSII, the oxygen-evolving complex (OEC), is an oxo-bridged Mn4Ca cluster arranged in a Mn3CaO4 cuboidal framework with a “dangling Mn” attached via a µ-oxo group. During turnover, the OEC progresses through a series of oxidation states, known as S states, in which the OEC is sequentially oxidized from the S0 state, the most reduced state, to the S4 state, the most oxidized state. The oxidation is accomplished by transfer of photonic energy to a special pair of chlorophylls in the reaction center (P680), resulting in a charge separation. Electron transfer occurs from excited P680* to a membrane-bound quinone, QA, via a pheophytin cofactor. In doing so, a stable charge separation is formed between P680+● and QA−●. The OEC is oxidized by the P680+● radical via a redox active tyrosine residue, Yz. With four sequential oxidation of the OEC from the S0 state to the S4 state, the OEC is capable of splitting water into dioxygen, four protons, and four electrons.
The enzyme photosystem II (PSII) catalyzes the light-driven oxidation of water in all of oxygenic photosynthesis. The active site of PSII, the oxygen-evolving complex (OEC), is an oxo-bridged Mn4Ca cluster arranged in a Mn3CaO4 cuboidal framework with a “dangling Mn” attached via a µ-oxo group. During turnover, the OEC progresses through a series of oxidation states, known as S states, in which the OEC is sequentially oxidized from the S0 state, the most reduced state, to the S4 state, the most oxidized state. The oxidation is accomplished by transfer of photonic energy to a special pair of chlorophylls in the reaction center (P680), resulting in a charge separation. Electron transfer occurs from excited P680* to a membrane-bound quinone, QA, via a pheophytin cofactor. In doing so, a stable charge separation is formed between P680+● and QA−●. The OEC is oxidized by the P680+● radical via a redox active tyrosine residue, Yz. With four sequential oxidation of the OEC from the S0 state to the S4 state, the OEC is capable of splitting water into dioxygen, four protons, and four electrons.
For the past three decades, our lab has probed the structure of the redox centers, the kinetics and yields of electron-transfer reactions, and the chemistry of water oxidation in PSII at the molecular level. We use EPR, mass spectrometry, flash oximetry, turnover measurements of oxygen evolution, and site-directed mutagenesis to monitor the photochemical events involved in catalysis and to obtain structural and mechanistic information on PSII. Currently, we are investigating the proton transfer and relay processes required for catalysis, the role of cations/anions in PSII, and the binding and reactions of inhibitors.
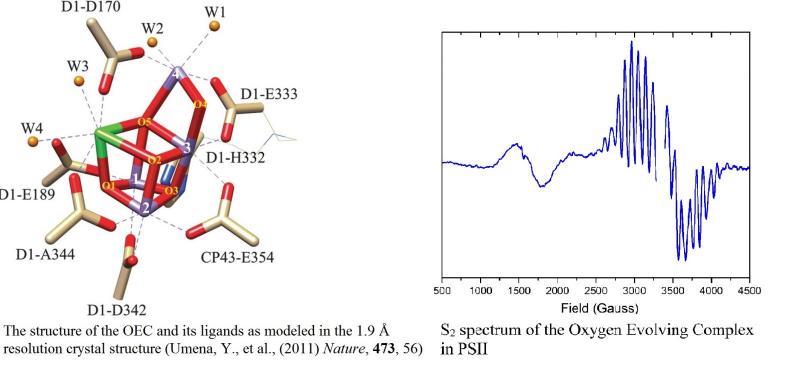
We are also studying the O-18 isotope effect of oxygen produced by PSII to gain insight about the origins of the Dole effect and to better understand the mechanism of oxygen evolution from PSII. Our research is primarily geared towards elucidating the structural and chemical properties of the OEC as it proceeds through the catalytic cycle.
References
“Water-Splitting Chemistry of Photosystem II”, James P. McEvoy and Gary W. Brudvig (2006) Chem. Rev. 106, 4455-4483.
“Quantum Mechanics/Molecular Mechanics Study of the Catalytic Cycle of Water Splitting in Photosystem II”, Eduardo M. Sproviero, José A. Gascón, James P. McEvoy, Gary W. Brudvig and Victor S. Batista (2008) J. Am. Chem. Soc. 130, 3428-3442.
“Chloride Regulation of Enzyme Turnover: Application to the Role of Chloride in Photosystem II”, Ravi Pokhrel, Iain L. McConnell and Gary W. Brudvig (2011) Biochemistry 50, 2725-2734.
“Oxygen-Evolving Complex of Photosystem II: Correlating Structure with Spectroscopy”, Ravi Pokhrel and Gary W. Brudvig (2014) Phys. Chem. Chem. Phys. 16, 11812-11821.