Geopolitical issues and environmental concerns emphasize the need for clean, secure, non-fossil energy sources.1-5 Solar energy collection and storage is one of the most promising lines for development. In this context, a biomimetic approach offers hope.6-9
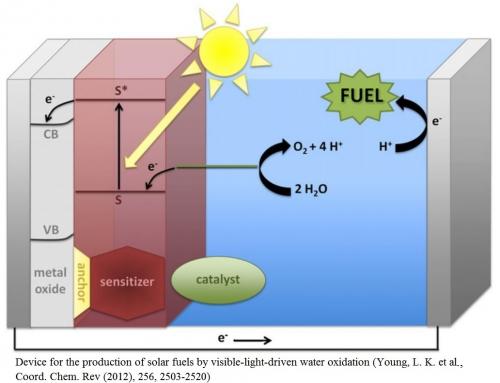
Photosynthesis provides a design for efficiently converting photons into a chemical fuel. In the biological system, PSII and PSI work in conjunction, with PSII removing electrons from water and PSI forming the reduced fuel product. Designing molecular-based systems with features that mimic the photosynthetic pathway is an important step towards an artificial photosynthetic system. The components of an artificial system must effectively combine the processes of light harvesting, photochemical charge separation, transfer of charges, and efficient catalysis. A challenging feature is the coupling of single-electron photochemical events to multi-electron catalytic reactions. Our interests include development of catalysts for water oxidation and proton reduction, molecular dyes, metal oxides, and surface-anchoring functional groups.
We aim to design, synthesize, optimize, and integrate the components of a device that converts solar energy into chemical fuels. Using ubiquitous water as the ultimate source of electrons in our photoelectrochemical device, and with an energy-rich chemical output, our design mimics Photosystem II and I, respectively.
 Towards the goal of a photo-driven water splitting device, we are developing dyes capable of mimicking features of PSII including light harvesting, energy transfer, and photo-induced electron transfer. These high-potential porphyrins and corroles become potent oxidants after photo-induced charge separation with a semiconductor, and are thermodynamically capable of oxidizing water. We are also developing dyes that mimic features of PSI. These low-potential corroles are capable of photo-induced electron injection into electrochemically-reducing semiconductor conduction bands for forming reduced fuels.
Towards the goal of a photo-driven water splitting device, we are developing dyes capable of mimicking features of PSII including light harvesting, energy transfer, and photo-induced electron transfer. These high-potential porphyrins and corroles become potent oxidants after photo-induced charge separation with a semiconductor, and are thermodynamically capable of oxidizing water. We are also developing dyes that mimic features of PSI. These low-potential corroles are capable of photo-induced electron injection into electrochemically-reducing semiconductor conduction bands for forming reduced fuels.
Recent efforts in our group have focused on the development of a high-potential porphyrin dye that is co-deposited on TiO2 nanoparticles together with a water-oxidation catalyst to give a water-splitting photoanode. The porphyrin sensitizer effectively extends light absorption by the anode into the visible region and the photoexcited dye injects electrons into the conduction band of TiO2. The limitations of this system are being assessed and re-designed to increase efficiency and stability.
References
1. IPCC, 2007: Summary for Policy Makers, Cambridge University Press, Cambridge,United Kingdom and New York, NY, U.S.A., 2007.
2. Directing Matter and Energy: Five Challenges for Science and the Imagination,U.S. Dep. Energy, Washington, D. C., December, 2007.
3. New Sciences for a Secure and Sustainable Energy Future, U.S. Dep. Energy, Washington, D. C., December, 2008.
4. Basic Research Needs: Catalysis For Energy, U.S. Dep. Energy, Washington, D.C., August, 2007.
5. Int. Energy Outlook 2009, U.S. Dep. Energy, Washington, D. C., May, 2009.
6. J. P. McEvoy and G. W. Brudvig, Chem. Rev. (Washington, DC, United States), 2006, 106, 4455-4483.
7. M. Hambourger, G. F. Moore, D. M. Kramer, D. Gust, A. L. Moore and T. A.Moore, Chem. Soc. Rev., 2009, 38, 25-35.
8. I. McConnell, G. Li and G. W. Brudvig, Chem. Biol. (Cambridge, MA, United States), 2010, 17, 434-447.
9. G. F. Moore and G. W. Brudvig, Annu. Rev. Condens. Matter Phys., 2011, DOI:10.1146/annurev-conmatphys-062910-140503, 2:2.1-2.25.